Colorful Science Investigation: Salt + Ice Hearts
Continuing our fun with ice, this time we combine science with art. Salt, ice hearts, and paint make for a very colorful science investigation. I thought the hearts would be a lovely addition for Valentine’s Day this week.
A Colorful Science Investigation
I remember trying this activity as a child: adding salt to ice and watching it melt. It’s a science lesson all on its own. As much as my 3 year old loves water play, he loves paint even more. This activity combines a science investigation with painting and water play. Do I need to tell you that my son was completely engrossed in this for over an hour? I’m sure he would have continued exploring if it wasn’t nap time.
Materials:
- Containers to use as ice molds – the taller the better {I used a large heart-shaped container and a small heart-shaped silicone cupcake mold.}
- Paint {We used food coloring and diluted acrylic paint. I have also seen it done with liquid watercolors.}
- Pan/rimmed container to hold the ice.
- Droppers
- Towels
- Paint clothes
How to Prepare for the Investigation
Add water to your containers/molds. Allow to freeze completely.
If you’re using food coloring, add several drops of food coloring to 1/2 cup water. {We also ended up adding straight food coloring to the ice to see the colors better. This is why I recommend using paint unless you just have an excess of food coloring you want to rid yourself of.}
If you’re using acrylic paint, dilute the paint with a little bit of water. {It’s interesting to note that the paint froze on top of the ice.}
If you’re using liquid watercolors, there’s really nothing to prepare except setting out the paint in containers.
How to Conduct the Investigation
- Place the ice in a large pan or rimmed container.
- Sprinkle salt on the top of the ice. The ice will begin to melt.
- Fill your dropper with colored water.
- Add colored water to the ice. You’ll notice the water runs through the crevices in the ice.
- Continue until the ice melts and your child asks for more.
Questions to Ask During the Investigation
What is happening to the ice? {There is water around it. It is melting.}
Why is it melting? {Water can only stay frozen when it’s really cold.}
What does the salt do? {Cracks the ice. Causes the ice to melt faster.}
What happens when you add colored water? {The water travels through the holes in the ice.}
You can also talk about color mixing. What color do we get when you add blue water to red water? {purple}
Our Investigation
In the picture below, we added food coloring straight to the ice. You can see how the color travels through the crevices.
Here you can see the crevices created by the salt and blue colored water.
We had already colored ice hearts leftover from our ice painting. Aiden asked me to save them in the freezer. They were frozen in this tower shape.
We added salt, colored water, and straight food coloring to them, too.
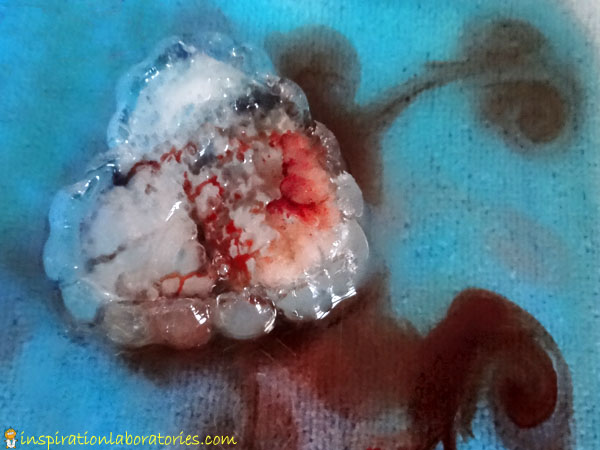
I brought out a large frozen heart {made from a heart-shaped plastic bowl}. After adding salt to the top, Aiden added dropper fulls of diluted acrylic paint. He made sure to cover every inch of the heart with the watered down paint.
The acrylic paint acted differently than the food coloring. It coated the crevices a bit more, so it was easier to see them.
The Science Behind It
Salt lowers the freezing point of water. Water normally freezes at 32°F (0°C). If you add salt, water won’t freeze at that temperature. It will actually freeze at a lower temperature. So how does salt cause ice to melt faster? When ice melts it usually takes a while because the water will melt and refreeze several times as the molecules are moving about. Salt disrupts the hydrogen bonds that hold the water molecules together. When the ice melts, the salt prevents it from refreezing. This causes the ice to melt faster.
More Valentine’s Day Ideas
- Frozen vinegar hearts are a great science idea perfect for Valentine’s Day.
- Make secret messages with baking soda.
- Look through our past Valentine’s Day ideas here.
Connect with Inspiration Laboratories on Twitter, Google+, Pinterest, or Facebook . You can also subscribe to posts by e-mail.
Linking up here.
Disclosure: This post contains affiliate links for Discount School Supply. See disclosure policy for more info.







Leave a Reply