Valentine Candy Science: Dissolving Hearts Experiment {with Printable}
Looking for science ideas for Valentine’s Day? Or maybe you need to find a use for all of that potential extra candy. Try your hand at some candy science with this dissolving hearts experiment. There’s even a printable for data collection.
Valentine Candy Science: Dissolving Hearts
This is a comparison experiment. We will be comparing how fast the candy hearts dissolve in different temperatures of water.
Materials:
- candy hearts
- containers {we used silicone cupcake liners}
- water of various temperatures
- timer/clock
Procedure:
- Fill containers with equal amounts of water at your different temperatures. We had 2 containers for each of our 3 temperatures {34°F, 72°F, and 120°F}.
- Make a prediction. Which temperature will dissolve the candy heart the fastest? Any ideas why?
- Add a candy heart to each of the containers.
- Start your timer and/or record the start time.
- Observe what happens.
- Check your experiment every so often and note any changes.
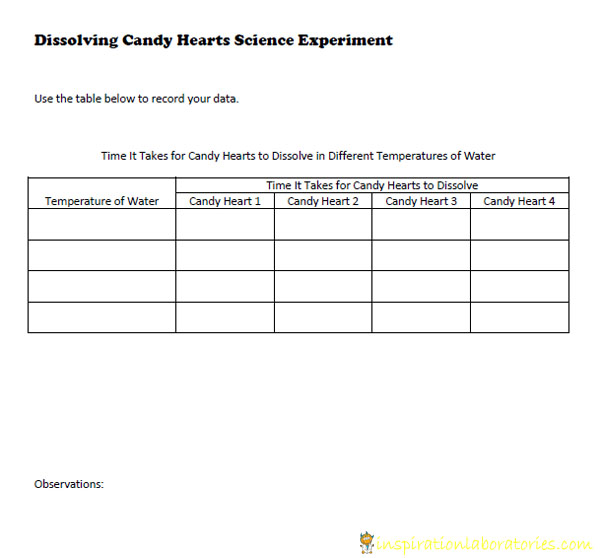
- Record the amount of time it takes for each candy heart to dissolve. {Use this handy data table.}
Our Observations
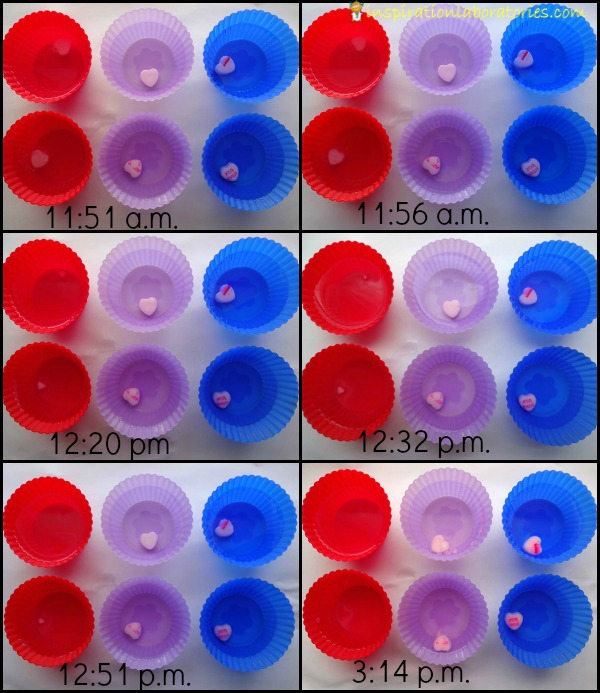
The picture below shows what we saw over time. Red cups contained hot water, purple had room temperature water, and blue contained cold water.
As expected, the candy hearts dissolved in the warmest water first. The candy hearts placed in room temperature water and cold water did not change much over the course of our experiment.
You’ll notice the latest time we checked on our experiment was 3:14 pm. When we checked on the candy hearts again that evening around 9pm, we noticed that two of the containers were knocked over and two of the hearts were missing {we suspect our dog as the likely culprit}. Not to worry, there was still one heart in a purple container and one in a blue container. The experiment can continue.
In the morning, all of the containers were knocked over and the remaining two hearts were missing…
Aiden had no desire to reset the experiment and try again. He wanted to mix together more baking soda and vinegar. Oh well, maybe we’ll try to finish it again another time. You’ll have to let us know which one dissolves next – the room temperature water or the cold water.
Experiment Printable
Download this printable to record your observations. Use the data table to organize your findings.
Click to download the pdf file: Dissolving Candy Hearts Science Experiment Printable
The Science Behind It
The candy hearts are made of mostly sugars. They are soluble in water {they will dissolve in water}. When the water touches the outside of the candy heart, tiny pieces of the candy {molecules really} dissolve into the water. Then, the next layer of the candy is exposed to the water. This continues until the entire candy heart is dissolved. Temperature affects how fast the candy dissolves. The higher the temperature, the faster the molecules move, so the faster the candy dissolves.
One interesting thing to note about our experiment is this. We only focused on the temperature of the water at the start of the experiment. The water temperature actually changes as time goes on. {The hot water will cool down, and the cold water will warm up. The room temperature water should remain fairly close as it will become the true temperature of your room.} Does this impact the results of the experiment? Possibly.
More Valentine’s Day Ideas
- Check out all of our candy science ideas with valentine hearts.
- Give baking soda and vinegar a Valentine’s Day theme with this frozen vinegar hearts idea.
- Look through our past Valentine’s Day ideas here. There are more activities still ahead!
Connect with Inspiration Laboratories on Twitter, Google+, Pinterest, or Facebook . You can also subscribe to posts by e-mail.
Linking up here.


Leave a Reply